University of Tübingen's researchers discover new antibiotic substance in the human nose
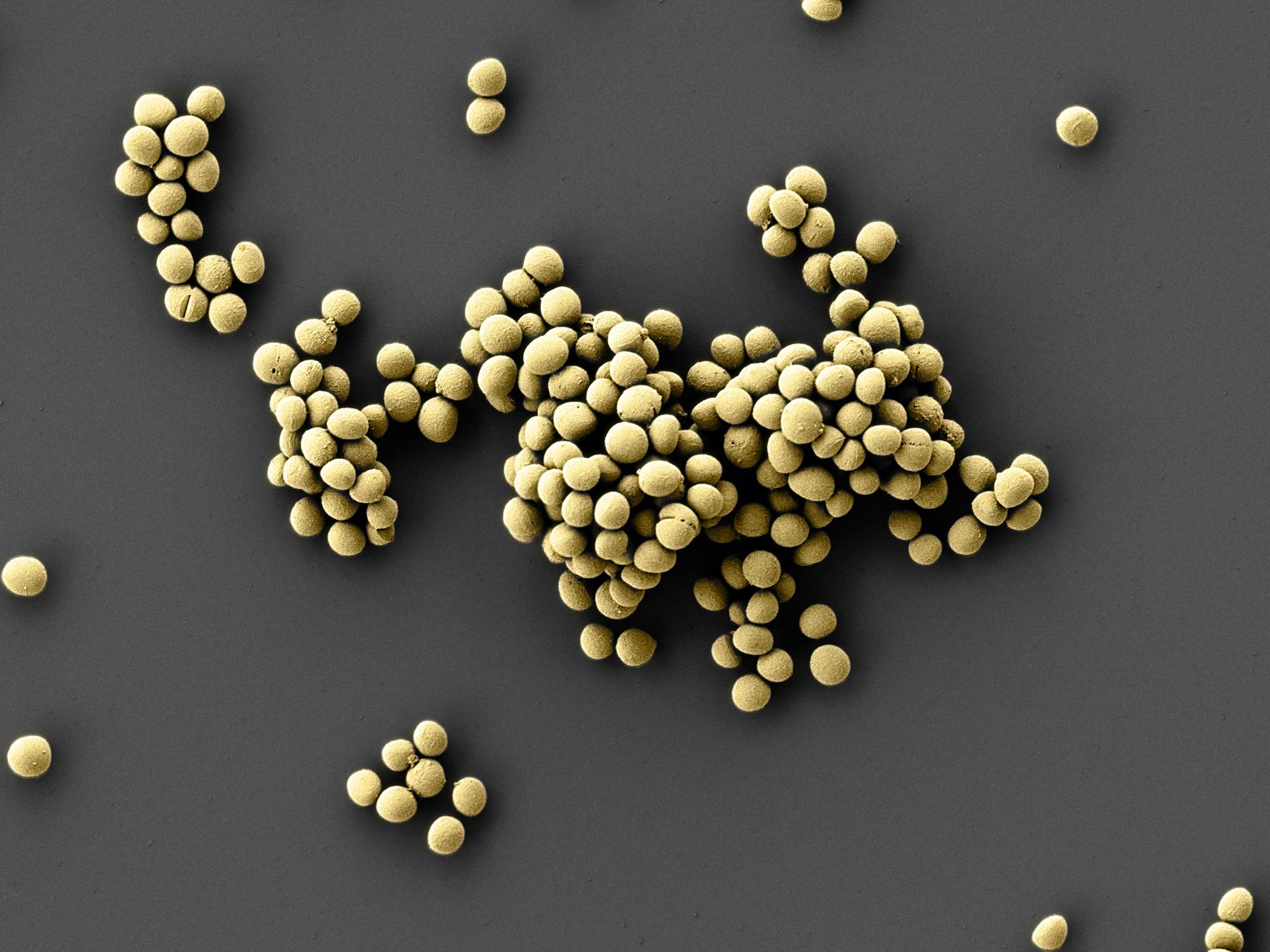
Copyright: Jeremiah Shuster, Tübingen Structural Microscopy Core Facility / Geomicrobiology / University of Tübingen
Coloration: Elke Neudert/ University of Tübingen
The human nose, skin and intestine are colonized by both benign and pathogenic bacteria. These microorganisms live together in what are called microbiomes. If the microbiome becomes unbalanced, pathogens can increase and we become ill. The bacterium Staphylococcus epidermidis occurs naturally in the dermal and nasal microbiomes of almost all humans. The newly-identified strain is believed to produce the active substance epifadin in order to survive against competing microorganisms. Epifadin not only works against the bacteria that are locally in competition with Staphylococcus epidermidis, but it is also effective against bacteria from other habitats such as the intestine and certain fungi. The researchers found that it is especially effective against the potential pathogens Staphylococcus aureus, a hospital-acquired infection which is particularly dangerous in antibiotic-resistant form (MRSA).
Back in 2016, the same working group - leaded by Dr Bernhard Krismer and Professor Andreas Peschel together with Professor Stephanie Grond and Professor Heike Brötz-Oesterhelt at the University of Tübingen - discovered an unknown antibiotic substance with an unique structure – Lugdunin. Epifadin is now the second discovery of this kind that this working group has made in the human microbiome.
Inside the lab
In experiments, the active substance epifadin reliably killed the pathogen Staphylococcus aureus, destroying hostile bacterial cells by damaging their cell membrane. The chemical structure of epifadin is extremely unstable and the substance is only active for just a few hours, so epifadin's effect is mainly local. This reduces the likelihood of collateral damage to the microbiome that is common with current treatments with broad-spectrum antibiotics. More research is needed to discover whether epifadin or its derivates can be used for therapy.

For instance, epifadin-producing Staphylococcus epidermidis might be colonized in the nasal mucosa and other places on our skin and thereby suppress the growth of pathogens such as Staphylococcus aureus. This could prevent bacterial infections – using natural means that our bodies already have.
How it all started
Researchers from the Cluster of Excellence “Controlling Microbes to Fight Infections” CMFI at the University of Tübingen tracked down the active substance and its structure ten years ago, when they first managed to isolate the strain. Complex natural substances such as epifadin are formed by microorganisms from single components using enzymes – process called ‘biosynthesis’. Initial attempts to reproduce this biosynthesis indicated early on that it was an extremely novel molecule. It took several years of close cooperation on chemical analysis and synthesis at the University of Tübingen’s Institute of Organic Chemistry before they succeeded in accumulating and storing the active substance in a way that enabled complete isolation of the pure substance.

photo by Jörg Jäger - University of Tübingen
Study leader Bernhard Krismer from the Interfaculty Institute of Microbiology and Infection Medicine Tübingen (IMIT) recalls:
“The data from the laboratory was extremely interesting, but difficult to interpret because of the instability. Despite the difficulties, I thought it was still worth continuing research into it. Tenacity and a high tolerance of frustration have finally led to success.”
Andreas Peschel, Professor of Microbiology at the University of Tübingen and speaker of the Cluster of Excellence CMFI, adds:
The development of new antibiotics has stagnated for decades. But we need them more than ever, because in recent years we’ve registered a rapid rise in multiresistant bugs worldwide. It is hard to get control of these infections and our reserve antibiotics no longer have such a strong effect. We urgently need new active substances and treatment methods.”
Follow-up studies will investigate the effect of the active substance on the basis of its structure. Here too, the perishability of epifadin makes extensive chemical and biological analysis more difficult. So, to begin with, they will use chemical synthesis in the laboratory to produce artificial molecules with a similar structure and antimicrobial effect as epifadin that are stable and easier to work with.
